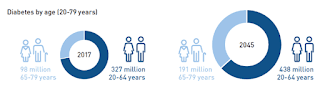Yellow fever
Smallpox Virus
Content
Smallpox, one of the biggest killers in history, is caused by a virus called variola. Variola causes a distinctive rash and is often lethal. The name variola comes from the Latin word for "spotted" and refers to the raised bumps that appear on the face and body of infected individuals. Although similar in name and in the formation of a rash, blisters, and scabs, variola belongs to a different virus family than the virus that causes the common childhood illness chicken pox. Variola is a member of the Poxvirus family of viruses. A close relative of variola within the Poxvirus family, called vaccinia, does not cause smallpox and is used as a vaccine for smallpox (in fact, the word vaccine comes from vaccinia virus). Vaccinia is also used in the laboratory to study this type of virus, because it is less hazardous to work with than the smallpox virus.
A virus related to variola called monkeypox virus recently made headlines when an outbreak occurred in the Midwestern region of the United States in 2003. This disease, also characterized by a rash and blisters, was the first monkeypox outbreak in the Western Hemisphere. Monkeypox virus sickened about 70 people. The cause was traced to prairie dogs that had been infected by imported African rodents at a pet distribution center. Fortunately, this disease was not as deadly as smallpox.
People generally become infected with the smallpox virus by breathing in virus droplets following exposure to infected individuals or by direct contact with infected fluids or contaminated objects. An unusual property of the smallpox virus is that it only infects humans and not animals and insects (this property was instrumental in the eradication of smallpox). After exposure to the virus, it usually takes one to two weeks before a person becomes ill and a rash and fever develop. At this point, the person is highly contagious and remains contagious until all scabs fall off after about three weeks. About 30% of infected people die from smallpox infection. People who recover from the infection are often left with permanent scars and sometimes blindness.
By some estimates, smallpox has been responsible for more deaths over the centuries than all other infectious diseases combined. A worldwide immunization program was instituted decades ago and has led to the elimination of smallpox as a human health threat. This has been one of the greatest success stories in medicine. In the United States, the last confirmed case of smallpox occurred in 1949, and worldwide the last recorded case of naturally occurring smallpox occurred in Somalia in 1977. In 1980, the World Health Organization formally declared that smallpox had been eradicated. There are currently only two official laboratory stockpiles of the smallpox virus in the world – one housed at the Centers for Disease Control and Prevention in Atlanta and the other at a research facility in Russia.
Deadly Diseases
Of all the diseases ever suffered by humans, smallpox is the only one to be completely eradicated from the face of the Earth. The few known remaining samples are under heavy guard in research labs in Russia and the United States.
The campaign to eradicate smallpox ended in 1980 and is one of the greatest triumphs of global public health. In that year, the World Health Organization announced that the disease known medically as variola major had been eliminated in its last pockets of infection, India, Bangladesh, and Africa.
In its natural form, smallpox was transmitted from person to person by air through tiny droplets of saliva, and thus found particularly fertile territory in crowded cities and along trade and travel routes. The disease began with high fever and body aches. The infected person was most contagious when a rash appeared. But the hallmark of smallpox was the pus-filled swellings of the skin that left telltale scars on those fortunate enough to survive. Many victims, however, lost their sight.
Historically, 30 percent of cases progressed to death. The end was often painful as the skin lesions spread into every bodily opening, including the mouth and eyes. When these lesions blackened and peeled off, they emitted a sweet and pungent odor known as the "smallpox smell."
The disease originated more than 3,000 years ago in Africa or China and became epidemic in Europe during the Middle Ages. The virus was so prevalent and lethal that in some societies, parents didn't name their children until they had survived a bout with the virus. Smallpox was the single deadliest disease during the 18th century, striking commoners and royalty alike. Luis I of Spain (1707-1724), Peter II of Russia (1715-1730), and Louis XV of France (1710-1774) all succumbed to the disease. In London, one-third of the population carried the pockmark scars from the disease.
Smallpox came to the New World in the late 1400s and early 1500s, decimating native populations in the West Indies and continental Americas. During the French and Indian War (1754-1763), the British intentionally gave smallpox-contaminated blankets to the Indians, resulting in an epidemic with a 50 percent mortality rate.
The story of humankind's triumph over the disease gained momentum with the experiments of an English country doctor, Edward Jenner. In 1796 he made the crucial observation that farmhands and dairy maids who contracted cowpox from cows never came down with the similar but more virulent smallpox. He reasoned that inducing a mild infection of cowpox in people might protect them from getting smallpox.
In what would today be considered an unethical experiment, Jenner tested his theory on an 8-year-old boy by introducing into his skin pus from a woman infected with cowpox. Six weeks later he made two incisions in the boy's arm and inserted smallpox pus. The boy did not come down with the disease. Jenner called his procedure a vaccination, from the Latin vacca, for "cow."
During the next two centuries, vaccinations worldwide helped reduce the incidence of smallpox. The disease was susceptible to eradication because it is a uniquely human disease with no known reservoirs in the animal or insect worlds. The last person naturally infected with smallpox was a hospital cook in Somalia in 1977. Once a routine vaccination for children in America, the practice ended with the eradication of the disease in 1980.
Unfortunately, the fear of smallpox remains palpable. Terrorist attacks in the 21st century have raised concerns that some of the research stockpile of the smallpox virus may have been sold to nations or groups who might use it as a biological weapon. Under optimum conditions, the fragile smallpox virus could survive 24 hours after being sprayed in aerosol form. Traces of the virus would be gone by the time symptoms of the disease appeared in people 10 to 12 days later.
The United States government has responded with a nationwide preparedness plan, with enough vaccine stored to inoculate the entire American population. For this plan to be effective, however, it would be critical to vaccinate promptly anyone who had come into contact with an infected person. The Centers for Disease Control has emergency "Push-Pak" supplies that can be flown quickly to any part of the country.
Return to Deadly Diseases
Poxvirus Tropism
Morens, D. M., Folkers, G. K. & Fauci, A. S. The challenge of emerging and re-emerging infectious diseases. Nature 430, 242–249 (2004).
Finlay, B. B., See, R. H. & Brunham, R. C. Rapid response research to emerging infectious diseases: lessons from SARS. Nature 2, 602–607 (2004).
Smith, G. L. & McFadden, G. Smallpox: anything to declare? Nature Rev. Immunol. 2, 521–528 (2002).
Tucker, J. B. Scourge: The Once and Future Threat of Smallpox (Atlantic Monthly Press, New York, 2001).
Alibek, K. Biohazard (Random House, New York, 1999).
Harrison, S. C. Et al. Discovery of antivirals against smallpox. Proc. Natl Acad. Sci. USA 101, 11178–11192 (2004).
Bray, M. & Roy, C. J. Antiviral prophylaxis of smallpox. J. Antimicrob. Chemother. 54, 1–5 (2004).
Lewis-Jones, S. Zoonotic poxvirus infection in humans. Curr. Opin. Infect. Dis. 17, 81–89 (2004). A useful summary of the poxviruses that can zoonotically infect man, which indicates which of these infections are clinically important.
Frey, S. E. & Belshe, R. B. Poxvirus zoonoses ? Putting pocks into context. N. Engl. J. Med. 350, 324–327 (2004).
Esposito, J. J. & Fenner, F. In Fields Virology 4th edn (eds Knipe, D. M. & Howley, P. M.) 2885–2921 (Lippincott Williams & Wilkins, Philadelphia, 2001).
Reed, K. D. Et al. The detection of monkeypox in humans in the western Hemisphere. N. Engl. J. Med. 350, 342–350 (2004).
Di Giulio, D. B. & Eckburg, P. B. Human monkeypox: an emerging zoonosis. Lancet Infect. Dis. 4, 15–25 (2004).
Hutin, Y. J. F. Et al. Outbreak of human monkeypox, Democratic Republic of Congo, 1996–1997. Emerg. Infect. Dis. 7, 434–438 (2001).
Meyer, H. Et al. Outbreaks of disease suspected of being due to human monkeypox virus infection in the Democratic Republic of Congo in 2001. J. Clin. Microbiol. 40, 2919–2921 (2002).
Breman, J. G. In Emerging Infections (eds Schield, W. M., Craig, W. A. & Hughes, J. M.) 45–67 (ASM Press, Washington DC, 2000).
Guarner, J. Et al. Monkeypox transmission and pathogenesis in prairie dogs. Emerg. Infect. Dis. 10, 426–431 (2004).
Fenner, F. & Ratcliffe, F. N. Myxomatosis (Cambridge Univ. Press, UK, 1965).
Sypula, J., Wang, F., Ma, Y., Bell, J. & McFadden, G. Myxoma virus tropism in human tumor cells. Gene Ther. Mol. Biol. 8, 103–114 (2004).
Dimitrov, D. S. Virus entry: molecular mechanisms and biomedical applications. Nature Rev. Microbiol. 2, 109–122 (2004).
Smith, A. E. & Helenius, A. How viruses enter animal cells. Science 304, 237–241 (2004).
Lalani, A. S. Et al. Use of chemokine receptors by poxviruses. Science 286, 1968–1971 (1999).
Eppstein, D. A. Et al. Epidermal growth factor receptor occupancy inhibits vaccinia virus infection. Nature 318, 663–665 (1985).
Johnston, J. B. Et al. Role of the serine–threonine kinase PAK-1 in myxoma virus replication. J. Virol. 77, 5877–5888 (2003).
Seet, B. T. Et al. Poxviruses and immune evasion. Annu. Rev. Immunol. 21, 377–423 (2003). Summarizes the known immunomodulator and host-range gene families found in poxviruses, and documents the members that can be encoded by different poxviruses.
Moss, B. In Fields Virology (eds Knipe, D. M. & Howley, P. M.) 2849–2883 (Lippincott Williams & Wilkins, Philadelphia, 2001).
Upton, C., Slack, S., Hunter, A. L., Ehlers, A. & Roper, R. L. Poxvirus orthologous clusters: toward defining the minimum essential poxvirus genome. J. Virol. 77, 7590–7600 (2003).
McLysaght, A., Baldi, P. F. & Gaut, B. S. Extensive gene gain associated with adaptive evoluton of poxviruses. Proc. Natl Acad. Sci. USA 100, 15655–15660 (2003).
Gubser, C., Hue, S., Kellam, P. & Smith, G. L. Poxvirus genomes: a phylogenetic analysis. J. Gen. Virol. 85, 105–117 (2004).
Johnston, J. B. & McFadden, G. Technical knockout: understanding poxvirus pathogenesis by selectively deleting viral immunomodulatory genes. Cell. Microbiol. 9, 695–705 (2004).
Turner, P. C. & Moyer, R. W. Poxvirus immune modulators: functional insights from animal models. Virus Res. 88, 35–53 (2002).
Smith, S. A. & Kotwal, G. J. Immune response to poxvirus infections in various animals. Crit. Rev. Microbiol. 28, 149–185 (2002).
Smith, G. L., Vanderplasschen, A. & Law, M. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 83, 2915–2931 (2002).
Vanderplasschen, A., Hollinshead, M. & Smith, G. L. Intracellular and extracellular vaccinia virions enter cells by different mechanisms. J. Gen. Virol. 79, 877–887 (1998).
Vanderplasschen, A. & Smith, G. L. A novel virus-binding assay using confocal microscopy: Demonstration that the intracellular and extracellular vaccinia virions bind to different cellular receptors. J. Virol. 71, 4032–4041 (1997).
Locker, J. K. Et al. Entry of the two infectious forms of vaccinia virus at the plasma membane is signaling-dependent for the IMV but not the EEV. Mol. Biol. Cell 11, 2497–2511 (2000).
Blasco, R., Sisler, J. R. & Moss, B. Dissociation of progeny vaccinia virus from the cell membrane is regulated by a viral envelope glycoprotein ? Effect of a point mutation in the lectin homology domain of the A34R gene. J. Virol. 67, 3319–3325 (1993).
Lin, C. L., Chung, C. S., Heine, H. G. & Chang, W. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J. Virol. 74, 3353–3365 (2000).
Hsiao, J. -C., Chung, C. -S. & Chang, W. Vaccinia envelope D8L protein binds to cell surface chondroitin sulfate and mediates intracellular mature virions adsorption to cells. J. Virol. 73, 8750–8761 (1999).
Chung, C. -S., Hsiao, J. -C., Chang, Y. -S. & Chang, W. A27L protein mediates vaccinia virus interaction with cell surface heparan sulfate. J. Virol. 72, 1577–1585 (1998).
Hsiao, J. -C., Chung, C. -S. & Chang, W. Cell surface proteoglycans are necessary for A27L protein-mediated cell fusion: identification of the N-terminal region of A27L protein as the glycosaminoglycan-binding domain. J. Virol. 72, 8374–8379 (1998).
Senkevich, T. G., Ward, B. M. & Moss, B. Vaccinia virus entry into cells is dependent on a virion surface protein encoded by the A28L gene. J. Virol. 78, 2357–2366 (2004). Describes the first poxviral protein (A28 of vaccinia virus) shown to be critical for the fusion/entry of poxviruses into mammalian cells.
Masters, J. Et al. Poxvirus infection rapidly activates tyrosine kinase signal transduction. J. Biol. Chem. 276, 48371–48375 (2001).
de Magalhaes, J. C. Et al. A mitogenic signal triggered at an early stage of vaccinia virus infection ? Implication of MEK/ERK and protein kinase in a virus multiplication. J. Biol. Chem. 276, 38353–38360 (2001).
Andrade, A. A. Et al. The vaccinia virus-stimulated mitogen-activated protein kinase (MAPK) pathway is required for virus multiplication. Biochem. J. 381, 437–446 (2004).
Greber, U. F. Signaling in viral entry. Cell. Mol. Life Sci. 59, 608–626 (2002).
Boehme, K. W. & Compton, T. Innate sensing of viruses by Toll-like receptors. J. Virol. 78, 7867–7873 (2004).
Bowie, A. Et al. A46R and A52R from vaccinia virus are antagonists of host IL-1 and Toll-like receptor signaling. Proc. Natl Acad. Sci. USA 97, 10162–10167 (2000).
Harte, M. T. Et al. The poxvirus protein A52R targets Toll-like receptor signaling complexes to suppress host defense. J. Exp. Med. 197, 343–351 (2003).
Broyles, S. S. Vaccinia virus transcripton. J. Gen. Virol. 84, 2293–2303 (2003).
Rosales, R., Sutter, G. & Moss, B. A cellular factor is required for transcription of vaccinia viral intermediate-stage genes. Proc. Natl Acad. Sci. USA 91, 3794–3798 (1994).
Rosales, R., Harris, N., Ahn, B. Y. & Moss, B. Purification and identification of a vaccinia virus-encoded intermediate stage promoter-specific transcription factor that has homology to eukaryotic transcription factor SII (TFIIS) and an additional role as a viral RNA polymerase subunit. J. Biol. Chem. 269, 14260–14267 (1994).
Sanz, P. & Moss, B. A new vaccinia virus intermediate transcription factor. J. Virol. 72, 6880–6883 (1998).
Gunasinghe, S. K., Hubbs, A. E. & Wright, C. F. A vaccinia virus late transcription factor with biochemical and molecular identity to a human cellular protein. J. Biol. Chem. 273, 27524–27530 (1998).
Wright, C. F., Hubbs, A. E., Gunasinghe, S. K. & Oswald, B. W. A vaccinia virus late transcription factor copurifies with a factor that binds to a viral late promoter and is complemented by extracts from uninfected HeLa cells. J. Virol. 72, 1446–1451 (1998).
Broyles, S. S., Liu, X., Zhu, M. & Kremer, M. Transcription factor YY1 is a vaccinia virus late promoter activator. J. Biol. Chem. 274, 35662–35667 (1999).
Katsafanas, G. C. & Moss, B. Vaccinia virus intermediate stage transcription is complemented by Ras-GTPase-activating protein SH3 domain-binding protein (G3BP) and cytoplasmic activation/proliferation-associated protein (p137) individually or as a heterodimer. J. Biol. Chem. 279, 52210–52217 (2004).
Smith, G. L., Murphy, B. J. & Law, M. Vaccinia virus motility. Annu. Rev. Microbiol. 57, 323–342 (2003).
Smith, G. L. & Law, M. The exit of vaccinia virus from infected cells. Virus Res. 106, 189–197 (2004).
Katz, E., Ward, B. M., Weisberg, A. S. & Moss, B. Mutations in the vaccinia virus A33R and B5R envelope proteins that enhance release of extracellular virions and eliminate formation of actin-containing microvilli without preventing tyrosine phosphorylation of the A36R protein. J. Virol. 77, 12266–12275 (2003).
Newsome, T. P., Scaplehorn, N. & Way, M. Src mediates a switch from microtubule- to actin-based motility of vaccinia virus. Science 306, 124–129 (2004).
Frischknecht, F. Et al. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature 401, 926–929 (1999).
McFadden, G., Pace, W. E., Purres, J. & Dales, S. Biogenesis of poxvirus: transitory expression of Molluscum contagiosum early functions. Virology 94, 297–313 (1979).
Li, Y., Yuan, S. & Moyer, R. W. The non-permissive infection of insect (Gypsy Moth) Ld-652 cells by vaccinia virus. Virology 248, 74–82 (1998).
Wali, A. & Strayer, D. S. Regulation of p53 gene expression by a poxviral transcription factor. Virology 224, 63–72 (1996).
Wali, A. & Strayer, D. S. Infection with vaccinia virus alters regulation of cell cycle progression. DNA Cell Biol. 18, 837–843 (1999).
Santos, C. R., Vega, F. M., Blanco, S., Barcia, R. & Lazo, P. A. The vaccinia virus B1R kinase induces p53 downregulation by an Mdm2-dependent mechanism. Virology 328, 254–265 (2004).
Guerra, S. Et al. Cellular gene expression survey of vaccinia virus infection of human HeLa cells. J. Virol. 77, 6493–6506 (2003).
Guerra, S. Et al. Microarray analysis reveals characteristic changes of host cell gene expression in response to attenuated modified vaccinia virus Ankara infection of human HeLa cells. J. Virol. 78, 5820–5834 (2004).
Senkevich, T. G. Et al. Genome sequence of a human tumorigenic poxvirus: prediction of specific host response-evasion genes. Science 273, 813–816 (1996).
Senkevich, T. G., Koonin, E. V., Bugert, J. J., Darai, G. & Moss, B. The genome of molluscum contagiosum virus: analysis and comparison with other poxviruses. Virology 233, 19–42 (1997).
Brown, M. Et al. Antigen gene transfer to cultured human dendritic cells using recombinant avipoxvirus vectors. Cancer Gene Ther. 6, 238–245 (1999).
Brown, M. Et al. Dendritic cells infected with recombinant fowlpox virus vectors are potent and long-acting stimulators of transgene-specific class 1 restricted T lymphocyte activity. Gene Ther. 7, 1680–1689 (2001).
Engelmayer, J. Et al. Mature dendritic cells infected with canarypox virus elicit strong anti-human immunodeficiency virus CD8+ and CD4+ T-cell responses from chronically infected individuals. J. Virol. 75, 2142–2153 (2001).
Ignatius, R. Et al. Canarypox virus-induced maturation of dendritic cells is mediated by apoptotic cell death and tumor necrosis factor-α secretion. J. Virol. 74, 11329–11338 (2000).
Drillien, R., Spehner, D. & Hanau, D. Modified vaccinia virus Ankara induces moderate activation of human dendritic cells. J. Gen. Virol. 85, 2167–2175 (2004).
Hurnlova, Z., Vokurka, M., Esteban, M. & Melkova, Z. Vaccinia virus induces apoptosis of infected macrophages. J. Gen. Virol. 83, 2821–2832 (2002).
Jenne, L., Hauser, C., Arrighi, J. F., Saurat, J. H. & Hugin, A. W. Poxvirus as a vector to transduce human dendritic cells for immunotherapy: abortive infection but reduced APC function. Gene Ther. 7, 1575–1583 (2000).
Bronte, V. Et al. Antigen expression by dendritic cells correlates with the therapeutic effectiveness of a model recombinant poxvirus tumor vaccine. Immunology 94, 3183–3188 (1997).
Engelmayer, J. Et al. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J. Immunol. 163, 6762–6768 (1999).
Drillien, R., Spehner, D., Bohbot, A. & Hanau, D. Vaccinia virus-related events and phenotypic changes after infection of dendritic cells derived from human monocytes. Virology 268, 471–481 (2000).
Hung, J. -J., Chung, C. -S. & Chang, W. Molecular chaperone Hsp90 is important for vaccinia virus growth in cells. J. Virol. 76, 1379–1390 (2002). One of the few studies to identify a host factor, in this case Hsp60, that is specifically required for efficient completion of the viral morphogenic pathway.
Sen, G. C. Viruses and interferon. Annu. Rev. Microbiol. 55, 255–281 (2001).
Samuel, C. E. Antiviral actions of interferons. Clin. Microbiol. Rev. 14, 778–809 (2001).
Katze, M. G., He, Y. & Gale, M. Jr. Viruses and interferon: a fight for supremacy. Nature Rev. Immunol. 2, 675–687 (2002).
Wang, F. Et al. Disruption of ERK 1/2 MAP kinase-dependent induction of type I interferon breaks myxoma virus species barrier. Nature Immunol. 5, 1266–1274 (2004). The first demonstration that the host species barrier for any poxvirus can be manipulated at the level of signal transduction, and shows that the induction of interferon by the infecting poxvirus is a crucial determinant of myxoma virus tropism.
Johnston, J. B., Nazarian, S. H., Natale, R. & McFadden, G. Myxoma virus infection of primary human fibroblasts varies with cellular age and is regulated by host interferon responses. Virology (in the press).
Ole, K. L. & Pickup, D. J. Cowpox virus and other members of the orthopoxvirus genus interfere with the regulation of NF-κB activation. Virology 288, 175–187 (2001).
Gil, J., Rullas, J., Alcami, J. & Estaban, M. MC159L protein from the poxvirus molluscum contagiosum virus inhibits NF-κ B activation and apoptosis induced by PKR. J. Gen. Virol. 82, 3027–3034 (2001).
Shisler, J. L. & Jin, X. -L. The vaccinia virus K1L gene product inhibits host NF-κBα degradation. J. Virol. 78, 3553–3560 (2004). Demonstrates that a poxvirus host-range factor, K1L of vaccinia, can affect the activation of an important antiviral pathway ? That of NF-κB.
DiPerna, G. Et al. Poxvirus protein N1L targets the I-κB kinase complex, inhibits signaling to NF-κB and IRF3 signaling by Toll-like receptors. J. Biol. Chem. 279, 36570–36578 (2004). Shows that a poxviral protein, N1L of vaccinia, might target the same host pathway as the host-range factor K1L, indicating that some host pathways are multiply targeted by poxviruses and that it is the summation of these modulations that affects tropism and host range.
Camus-Bouclainville, C. Et al. A virulence factor of myxoma virus colocalizes with NF-κB in the nucleus and interferes with inflammation. J. Virol. 78, 2510–2516 (2004).
Everett, H. & McFadden, G. Poxviruses and apoptosis: a time to die. Curr. Opin. Microbiol. 5, 395–402 (2002).
Shisler, J. L. & Moss, B. Immunology 102 at poxvirus U: avoiding apoptosis. Semin. Immunol. 13, 67–72 (2001).
Barry, M., Wasilenko, S. T., Stewart, T. L. & Taylor, J. M. Apoptosis regulator genes encoded by poxviruses. Prog. Mol. Subcell. Biol. 36, 19–37 (2004).
Everett, H. Et al. The myxoma poxvirus protein, M11L, prevents apoptosis by direct interaction with the mitochondrial permeability transition pore. J. Exp. Med. 196, 1127–1139 (2002).
Wasilenko, S. T., Stewart, T. L., Meyers, A. F. & Barry, M. Vaccinia virus encodes a previously uncharacterized mitochondrial-associated inhibitor of apoptosis. Proc. Natl Acad. Sci. USA 100, 14345–14350 (2003).
Wang, G. Et al. Myxoma virus M11L prevents apoptosis through constitutive interaction with Bak. J. Virol. 78, 7097–7111 (2004).
Fenner, F. & Sambrook, J. F. Conditional lethal mutants of rabbitpox virus II. Mutants (p) that fail to multiply in PK-2a cells. Virology 28, 600–609 (1966).
Sambrook, J. F., Padgett, B. L. & Tomkins, J. K. N. Conditional lethal mutants of rabbitpox virusI Isolation of host cell-dependent and temperature-dependent mutants. Virology 28, 592–599 (1966).
Gemmell, A. & Fenner, F. Genetic studies with mammalian poxviruses. III. White (u) mutants of rabbitpox virus. Virology 11, 219–235 (1960).
McClain, M. E. The host range and plaque morphology of rabbitpox virus (RPμ+) and its μ mutants on chick fibroblast, PK-2a, and L929 cells. Aust. J. Exp. Biol. Med. Sci. 43, 31–44 (1965).
McClain, M. E. & Greenland, R. H. Recombination between rabbitpox virus mutants in permissive and nonpermissive cells. Virology 25, 516–522 (1965).
Lake, J. R. & Cooper, P. D. Deletions of the terminal sequences in the genome of the white pock (μ) and host restricted (ρ) mutants of rabbitpox virus. J. Gen. Virol. 48, 135–147 (1980).
Moyer, R. W., Brown, G. D. & Graves, R. L. The white pock mutants of rabbit poxvirus. II. The early white pock (mu) host range (hr) mutants of rabbit poxvirus uncouple transcription and translation in nonpermissive cells. Virology 106, 234–249 (1980).
Ali, A. N., Turner, P. C., Brooks, M. A. & Moyer, R. W. The SPI-1 gene of rabbitpox virus determines host range and is required for hemorrhagic pock formation. Virology 202, 305–314 (1994).
Brooks, M. A., Ali, A. N., Turner, P. C. & Moyer, R. W. A rabbitpox virus serpin gene controls host range by inhibiting apoptosis in restrictive cells. J. Virol. 69, 7688–7698 (1995).
Shisler, J. L., Isaacs, S. N. & Moss, B. Vaccinia virus serpin-1 deletion mutant exhibits a host range defect charracterized by low levels of intermediate and late mRNAs. Virology 262, 298–311 (1999).
Macen, J. L. Et al. Differential inhibition of the Fas- and granule-mediated cytolysis pathways by the orthopoxvirus cytokine response modifier A/SPI-2 and SPI-1 protein. Proc. Natl Acad. Sci. USA 93, 9108–9113 (1996).
Moon, K. B., Turner, P. C. & Moyer, R. W. SPI-1-dependent host range of rabbitpox virus and complex formation with cathepain G is associated with serpin motifs. J. Virol. 73, 8999–9010 (1999).
Wallich, R., Simon, M. M. & Mullbacher, A. Virulence of mousepox virus is independent of serpin-mediated control of cellular cytotoxicity. Viral Immunol. 14, 71–81 (2001).
Kettle, S., Blake, N. W., Law, K. M. & Smith, G. L. Vaccinia virus serpins B13R (SPI-2) and B22R (SPI-1) encode M(r) 38.5 and 40K, intracellular polypeptides that do not affect virus virulence in a murine intranasal model. Virology 206, 136–147 (1995).
Legrand, F. A. Et al. Induction of potent humoral and cell-mediated immune responses by attenuated vaccinia virus vectors with deleted serpin genes. J. Virol. 78, 2770–2779 (2004).
Perkus, M. E. Et al. Vaccinia virus host range genes. Virology 179, 276–286 (1990). A classic paper summarizing the discovery that specific vaccinia host-range genes control the ability of poxviruses to infect mammalian cells of a given host species.
Drillien, Koehren, F. & Kirn, A. Host-range deletion mutant of vaccinia virus defective in human cells. Virology 111, 488–499 (1981).
Drillien, R., Spehner, D. & Kirn, A. Host range restriction of vaccinia virus in Chinese hamster ovary cells: relationship to shutoff of protein synthesis. J. Virol. 28, 843–850 (1978).
Hruby, D. E., Lynn, D. L., Condit, R. C. & Kates, J. R. Cellular differences in the molecular mechanisms of vaccinia virus host-range restriction. J. Gen. Virol. 47, 485–488 (1980).
Gillard, S., Spehner, D. & Drillien, R. Mapping of a vaccinia host range sequence by insertion into the viral thymidine kinase gene. J. Virol. 53, 316–318 (1985).
Gillard, S., Spehner, D., Drillien, R. & Kirn, A. Localization and sequence of a vaccinia virus gene required for multiplication in human cells. Proc. Natl Acad. Sci. USA 83, 5573–5577 (1986).
Ramsey-Ewing, A. & Moss, B. Apoptosis induced by a postbinding step of vaccinia virus entry into Chinese hamster ovary cells. Virology 242, 138–149 (1998).
Spehner, D., Gillard, S., Drillien, R. & Kirn, A. A cowpox virus gene required for multiplication in Chinese hamster ovary cells. J. Virol. 62, 1297–1304 (1988). Another classic paper showing that a specific gene, CHOhr (or CP77), is required for the replication of cowpox virus in CHO cells.
Oguiura, N., Spehner, D. & Drillien, R. Detection of a protein encoded by the vaccinia virus C7L open reading frame and study of its effect on virus multiplication in different cell lines. J. Gen. Virol. 74, 1409–1413 (1993).
Chen, W., Drillien, R., Spehner, D. & Buller, R. M. L. Restricted replication of ectromelia virus in cell culture correlates with mutation in virus-encoded host range genes. Virology 187, 433–442 (1992).
Ramsey-Ewing, A. & Moss, B. Restriction of vaccinia virus replication in CHO cells occurs at the stage of viral intermediate protein synthesis. Virology 206, 984–993 (1995).
Ink, B. S., Gilbert, C. S. & Evans, G. I. Delay of vaccinia virus-induced apoptosis in nonpermissive Chinese hamster ovary cells by the cowpox virus CHOhr and adenovirus E1B 19K genes. J. Virol. 69, 661–668 (1995).
Hsiao, J. -C., Chung, C. -S., Drillien, R. & Chang, W. The cowpox virus host range gene, CP77, affects phosphorylation of eIF2 α and vaccinia viral translation in apoptotic HeLa cells. Virology 329, 199–212 (2004).
Ramsey-Ewing, A. L. & Moss, B. Complementation of a vaccinia virus host-range K1L gene deletion by the nonhomologous CP77 gene. Virology 222, 75–86 (1996).