Ebola virus disease
Cerebral Adrenoleukodystrophy Pipeline Insight 2024, Featuring Key Players Minoryx Therapeutics, POXEL, Orpheris And Bluebird Bio
Company Logo
Dublin, April 17, 2024 (GLOBE NEWSWIRE) -- The "Cerebral Adrenoleukodystrophy - Pipeline Insight, 2024" has been added to ResearchAndMarkets.Com's offering.
The report provides comprehensive insights about 3+ companies and 3+ pipeline drugs in Cerebral Adrenoleukodystrophy pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
The report outlays comprehensive insights of present scenario and growth prospects across the indication. A detailed picture of the Cerebral Adrenoleukodystrophy pipeline landscape is provided which includes the disease overview and Cerebral Adrenoleukodystrophy treatment guidelines. The assessment part of the report embraces, in depth Cerebral Adrenoleukodystrophy commercial assessment and clinical assessment of the pipeline products under development. In the report, detailed description of the drug is given which includes mechanism of action of the drug, clinical studies, NDA approvals (if any), and product development activities comprising the technology, Cerebral Adrenoleukodystrophy collaborations, licensing, mergers and acquisition, funding, designations and other product related details.
Report Highlights
A better understanding of disease pathogenesis contributing to the development of novel therapeutics for Cerebral Adrenoleukodystrophy.
In the coming years, the Cerebral Adrenoleukodystrophy market is set to change due to the rising awareness of the disease, and incremental healthcare spending across the world; which would expand the size of the market to enable the drug manufacturers to penetrate more into the market.
The companies and academics that are working to assess challenges and seek opportunities that could influence Cerebral Adrenoleukodystrophy R&D. The therapies under development are focused on novel approaches to treat/improve the disease condition.
A detailed portfolio of major pharma players who are involved in fueling the Cerebral Adrenoleukodystrophy treatment market. Several potential therapies for Cerebral Adrenoleukodystrophy are under investigation. With the expected launch of these emerging therapies, it is expected that there will be a significant impact on the Cerebral Adrenoleukodystrophy market size in the coming years.
This in-depth analysis of the pipeline assets (in early-stage, mid-stage and late stage of development for the treatment of Cerebral Adrenoleukodystrophy) includes therapeutic assessment and comparative analysis. This will support the clients in the decision-making process regarding their therapeutic portfolio by identifying the overall scenario of the research and development activities.
Story continues
Cerebral Adrenoleukodystrophy Emerging Drugs Chapters
This segment of the Cerebral Adrenoleukodystrophy report encloses its detailed analysis of various drugs in different stages of clinical development, including phase II, I, preclinical and Discovery. It also helps to understand clinical trial details, expressive pharmacological action, agreements and collaborations, and the latest news and press releases.
Cerebral Adrenoleukodystrophy Emerging Drugs
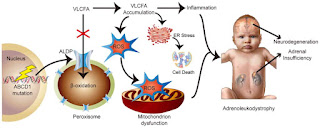
MIN-102: Minoryx TherapeuticsLeriglitazone (MIN-102) is a novel, orally bioavailable and selective PPAR gamma agonist with a potential best-in-class profile indicated for CNS diseases. It is one of the several metabolites of pioglitazone and has a demonstrated sufficient brain penetration and favorable safety profile in humans, allowing PPAR gamma engagement in the CNS above the level that can be safely achieved with pioglitazone and other glitazones. It showed robust preclinical proof-of-concept in animal models of multiple diseases by modulating pathways leading to mitochondrial dysfunction, oxidative stress, neuroinflammation, demyelination and axonal degeneration.
PXL770: POXEL SAPXL770 is a novel drug candidate that directly activates adenosine monophosphate-activated protein kinase (AMPK). AMPK is a central regulator of multiple metabolic pathways leading to the control of multiple metabolic pathways and reduced inflammation. The rationale for considering AMPK activators in ALD is based on several published findings that show links between AMPK and disease in both animals and humans (slide below). Based on these observations, PXL770 was evaluated in both the in vitro and in vivo ALD models (charts below). PXL770 suppressed elevated VLCFA levels in patient derived cells with an associated increase in expression of the compensatory ABCD2 transporter. PXL770 treatment of ABCD1 null mice also suppressed elevated VLCFA in spinal cord - and in brain and plasma (not shown). Additional experimental results included an observed improvement in neural histology and neuro-behavior in ABCD1 null mice following chronic PXL770 treatment.
Cerebral Adrenoleukodystrophy: Therapeutic Assessment
This segment of the report provides insights about the different Cerebral Adrenoleukodystrophy drugs segregated based on following parameters that define the scope of the report, such as:
Major Players in Cerebral Adrenoleukodystrophy
There are approx. 3+ key companies which are developing the therapies for Cerebral Adrenoleukodystrophy. The companies which have their Cerebral Adrenoleukodystrophy drug candidates in the most advanced stage, i.E. Phase II/III include, Minoryx Therapeutics.
Phases
This report covers around 3+ products under different phases of clinical development like:
Late stage products (Phase III)
Mid-stage products (Phase II)
Early-stage product (Phase I) along with the details of
Pre-clinical and Discovery stage candidates
Discontinued & Inactive candidates
Route of Administration
Cerebral Adrenoleukodystrophy pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as:
Inhalation
Inhalation/Intravenous/Oral
Intranasal
Intravenous
Intravenous/ Subcutaneous
NA
Oral
Oral/intranasal/subcutaneous
Parenteral
Subcutaneous
Molecule Type
Products have been categorized under various Molecule types such as:
Product Type
Cerebral Adrenoleukodystrophy: Pipeline Development Activities
The report provides insights into different therapeutic candidates in phase II, I, preclinical and discovery stage. It also analyses Cerebral Adrenoleukodystrophy therapeutic drugs key players involved in developing key drugs.
Pipeline Development Activities
The report covers the detailed information of collaborations, acquisition and merger, licensing along with a thorough therapeutic assessment of emerging Cerebral Adrenoleukodystrophy drugs.
Cerebral Adrenoleukodystrophy Report Insights
Cerebral Adrenoleukodystrophy Report Assessment
Key Questions
Current Treatment Scenario and Emerging Therapies:
How many companies are developing Cerebral Adrenoleukodystrophy drugs?
How many Cerebral Adrenoleukodystrophy drugs are developed by each company?
How many emerging drugs are in mid-stage, and late-stage of development for the treatment of Cerebral Adrenoleukodystrophy?
What are the key collaborations (Industry-Industry, Industry-Academia), Mergers and acquisitions, licensing activities related to the Cerebral Adrenoleukodystrophy therapeutics?
What are the recent trends, drug types and novel technologies developed to overcome the limitation of existing therapies?
What are the clinical studies going on for Cerebral Adrenoleukodystrophy and their status?
What are the key designations that have been granted to the emerging drugs?
Key Players
Minoryx Therapeutics
POXEL SA
Orpheris, Inc.
bluebird bio, Inc.
Key Products
For more information about this clinical trials report visit https://www.Researchandmarkets.Com/r/d650ff
About ResearchAndMarkets.ComResearchAndMarkets.Com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
CONTACT: CONTACT: ResearchAndMarkets.Com Laura Wood,Senior Press Manager press@researchandmarkets.Com For E.S.T Office Hours Call 1-917-300-0470 For U.S./ CAN Toll Free Call 1-800-526-8630 For GMT Office Hours Call +353-1-416-8900View comments
Vitamins & Supplements Center
Considering taking a vitamin or supplement to treat Adrenoleukodystrophy (Ald)? Below is a list of common natural remedies used to treat or reduce the symptoms of Adrenoleukodystrophy (Ald). Follow the links to read common uses, side effects, dosage details and read user reviews for the drugs listed below.
2 results found for Adrenoleukodystrophy (Ald)
Learn about User Reviews and read IMPORTANT information about user generated content
Conditions of Use and Important Information: This information is meant to supplement, not replace advice from your doctor or healthcare provider and is not meant to cover all possible uses, precautions, interactions or adverse effects. This information may not fit your specific health circumstances. Never delay or disregard seeking professional medical advice from your doctor or other qualified health care provider because of something you have read on WebMD. You should always speak with your doctor or health care professional before you start, stop, or change any prescribed part of your health care plan or treatment and to determine what course of therapy is right for you.
This copyrighted material is provided by Natural Medicines Comprehensive Database Consumer Version. Information from this source is evidence-based and objective, and without commercial influence. For professional medical information on natural medicines, see Natural Medicines Comprehensive Database Professional Version.© Therapeutic Research Faculty 2018.
Gene Redundancy And Pharmacological Gene Therapy: Implications For X-linked Adrenoleukodystrophy
Cooke, J., Nowak, M.A., Boerlijst, M. & Maynard-Smith, J. Evolutionary origins and maintenance of redundant gene expression during metazoan development. Trends Genet. 13, 360– 364 (1997).
Thomas, J.H. Thinking about genetic redundancy. Trends Genet. 9, 395–399 (1993).
Charache, S. Et al. Treatment of sickle cell anemia with 5-azacytidine results in increased fetal hemoglobin production and is associated with nonrandom hypomethylation of DNA around the gamma-delta-beta-globin gene complex. Proc. Natl. Acad. Sci. USA 80, 4842– 4846 (1983).
Dover, G.J. Et al. Hydroxyurea induction of hemoglobin F production in sickle cell disease: relationship between cytotoxicity and F cell production. Blood 67, 735–738 ( 1986).
Perrine, S.P. Et al. A short-term trial of butyrate to stimulate fetal-globin-gene expression in the beta-globin disorders [see comments]. N. Engl. J. Med. 328, 81–86 ( 1993).
Dover, G.J., Brusilow, S. & Charache, S. Induction of fetal hemoglobin production in subjects with sickle cell anemia by oral sodium phenylbutyrate. Blood 84, 339–343 (1994).
Tinsley, J.M. Et al. Amelioration of the dystrophic phenotype of mdx mice using a truncated utrophin transgene [see comments]. Nature 384, 349–353 (1996).
Roush, W. Backup gene may help muscles help themselves [news]. Science 276, 35 (1997).
Wanders, R.J. Et al. Direct demonstration that the deficient oxidation of very long chain fatty acids in X-linked adrenoleukodystrophy is due to an impaired ability of peroxisomes to activate very long chain fatty acids. Biochem. Biophys. Res. Commun. 153, 618– 624 (1988).
Lazo, O., Contreras, M., Bhushan, A., Stanley, W. & Singh, I. Adrenoleukodystrophy: impaired oxidation of fatty acids due to peroxisomal lignoceroyl-CoA ligase deficiency. Arch. Biochem. Biophys. 270, 722–728 (1989).
Moser, H.W., Smith, K.D. & Moser, A.B. In The Metabolic Basis of Inherited Disease 7th edn. (eds. Scirver, C.R., Baeudet, A.L., Sly, W.S. & Valle, D.) 4256–4300 (McGraw Hill, New York, 1995).
Powers, J.M. Adreno-leukodystrophy (adreno-testiculo-leukomyelo-neuropathic-complex). Clin. Neuropathol. 4, 181–199 (1985).
Mosser, J. Et al. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature 361, 726–730 (1993).
Kemp, S. Et al. Identification of a two base pair deletion in five unrelated families with adrenoleukodystrophy: a possible hot spot for mutations. Biochem. Biophys. Res. Commun. 202, 647– 653 (1994).
Kok, F. Et al. Mutational analysis of patients with X-linked adrenoleukodystrophy. Hum. Mutat. 6, 104–115 (1995).
Mosser, J. Et al. The gene responsible for adrenoleukodystrophy encodes a peroxisomal membrane protein. Hum. Mol. Genet. 3, 265 –271 (1994).
Ligtenberg, M.J. Et al. Spectrum of mutations in the gene encoding the adrenoleukodystrophy protein. Am. J. Hum. Genet. 56, 44– 50 (1995).
Feigenbaum, V. Et al. Mutational and protein analysis of patients and heterozygous women with X-linked adrenoleukodystrophy. Am. J. Hum. Genet. 58, 1135–1144 (1996).
Krasemann, E.W., Meier, V., Korenke, G.C., Hunneman, D.H. & Hanefeld, F. Identification of mutations in the ALD-gene of 20 families with adrenoleukodystrophy/adrenomyeloneuropathy. Hum. Genet. 97, 194–197 (1996).
Cartier, N. Et al. Retroviral-mediated gene transfer corrects very-long-chain fatty acid metabolism in adrenoleukodystrophy fibroblasts. Proc. Natl. Acad. Sci. USA 92, 1674–1678 (1995).
Braiterman, L.T. Et al. Suppression of peroxisomal membrane protein defects by peroxisomal ATP binding cassette (ABC) proteins. Hum. Mol. Genet. 7, 239–247 ( 1998).
Lombard-Platet, G., Savary, S., Sarde, C.O., Mandel, J.L. & Chimini, G. A close relative of the adrenoleukodystrophy (ALD) gene codes for a peroxisomal protein with a specific expression pattern. Proc. Natl. Acad. Sci. USA 93, 1265– 1269 (1996).
Holzinger, A., Kammerer, S., Berger, J. & Roscher, A.A. CDNA cloning and mRNA expression of the human adrenoleukodystrophy related protein (ALDRP), a peroxisomal ABC transporter. Biochem. Biophys. Res. Commun. 239, 261–264 (1997).
Kamijo, K., Taketani, S., Yokota, S., Osumi, T. & Hashimoto, T. The 70-kDa peroxisomal membrane protein is a member of the Mdr (P-glycoprotein)-related ATP-binding protein superfamily. J. Biol. Chem. 265, 4534–4540 (1990).
Gartner, J., Moser, H. & Valle, D. Mutations in the 70K peroxisomal membrane protein gene in Zellweger syndrome. Nature Genet. 1, 16–23 (1992).
Shani, N., Jimenez-Sanchez, G., Steel, G., Dean, M. & Valle, D. Identification of a fourth half ABC transporter in the human peroxisomal membrane. Hum. Mol. Genet. 6, 1925–1931 ( 1997).
Holzinger, A., Kammerer, S. & Roscher, A.A. Primary structure of human PMP69, a putative peroxisomal ABC-transporter. Biochem. Biophys. Res. Commun. 237 , 152–157 (1997).
Boles, D.J., Craft, D.A., Padgett, D.A., Loria, R.M. & Rizzo, W.B. Clinical variation in X-linked adrenoleukodystrophy: fatty acid and lipid metabolism in cultured fibroblasts. Biochem. Med. Metab. Biol. 45, 74–91 (1991).
Lu, J.F. Et al. A mouse model for X-linked adrenoleukodystrophy. Proc. Natl. Acad. Sci. USA 94, 9366– 9371 (1997).
Loes, D.J. Et al. Childhood cerebral form of adrenoleukodystrophy: short-term effect of bone marrow transplantation on brain MR observations. AJNR Am. J. Neuroradiol. 15, 1767– 1771 (1994).
Moser, H.W. Adrenoleukodystrophy: phenotype, genetics, pathogenesis and therapy. Brain 120, 1485–1508 ( 1997).
Poulos, A., Gibson, R., Sharp, P., Beckman, K. & Grattan-Smith, P. Very long chain fatty acids in X-linked adrenoleukodystrophy brain after treatment with Lorenzo's oil. Ann. Neurol. 36, 741–746 (1994).
Rasmussen, M., Moser, A.B., Borel, J., Khangoora, S. & Moser, H.W. Brain, liver, and adipose tissue erucic and very long chain fatty acid levels in adrenoleukodystrophy patients treated with glyceryl trierucate and trioleate oils (Lorenzo's oil). Neurochem. Res. 19, 1073–1082 ( 1994).
Maestri, N.E., Brusilow, S.W., Clissold, D.B. & Bassett, S.S. Long-term treatment of girls with ornithine transcarbamylase deficiency. N. Engl. J. Med. 335, 855–859 (1996).
Carstea, E.D., Murray, G.J. & O'Neill, R.R. Molecular and functional characterization of the murine glucocerebrosidase gene. Biochem. Biophys. Res. Commun. 184, 1477–1483 (1992).
Deng, G., Liu, G., Hu, L., Gum, J.R., Jr. & Kim, Y.S. Transcriptional regulation of the human placental-like alkaline phosphatase gene and mechanisms involved in its induction by sodium butyrate. Cancer Res. 52, 3378–3383 (1992).
Fregeau, C.J., Helgason, C.D. & Bleackley, R.C. Two cytotoxic cell proteinase genes are differentially sensitive to sodium butyrate. Nucleic Acids Res. 20 , 3113–3119 (1992).
Liu, L., Hudgins, W.R., Miller, A.C., Chen, L.C. & Samid, D. Transcriptional upregulation of TGF-alpha by phenylacetate and phenylbutyrate is associated with differentiation of human melanoma cells. Cytokine 7, 449– 456 (1995).
Albet, S. Et al. Fenofibrate differently alters expression of genes encoding ATP-binding transporter proteins of the peroxisomal membrane. FEBS Lett. 405, 394–397 (1997).
Rubenstein, R.C., Egan, M.E. & Zeitlin, P.L. In vitro pharmacologic restoration of CFTR-mediated chloride transport with sodium 4-phenylbutyrate in cystic fibrosis epithelial cells containing delta F508-CFTR. J. Clin. Invest. 100, 2457–2465 (1997).
Rubenstein, R.C. & Zeitlin, P.L. A pilot clinical trial of oral sodium 4-phenylbutyrate (Buphenyl) in deltaF508-homozygous cystic fibrosis patients: partial restoration of nasal epithelial CFTR function. Am. J. Respir. Crit. Care. Med. 157, 484 –490 (1998).
Watkins, P.A. Et al. Altered expression of ALDP in X-linked adrenoleukodystrophy. Am. J. Hum. Genet. 57, 292– 301 (1995).
Kemp, S. Et al. ALDP expression in fibroblasts of patients with X-linked adrenoleukodystrophy. J. Inherit. Metab. Dis. 19, 667– 674 (1996).
Reddy, J.K., Suga, T., Mannaerts, G.P., Lazarow, P.B. & Subramani, S. In Annals of the New York Academy of Sciences Vol. 804 (eds. Boland, B., Cullinan, J., Cullinan, D.M. & Garry, M.L.) 801 (New York Academy of Sciences, New York, 1996).
Pineau, T. Et al. Activation of a human peroxisome proliferator-activated receptor by the antitumor agent phenylacetate and its analogs. Biochem. Pharmacol. 52, 659–667 ( 1996).
Aoyama, T., Souri, M., Kamijo, T., Ushikubo, S. & Hashimoto, T. Peroxisomal acyl-coenzyme A oxidase is a rate-limiting enzyme in a very-long-chain fatty acid beta-oxidation system. Biochem. Biophys. Res. Commun. 201, 1541– 1547 (1994).
Fouquet, F. Et al. Expression of the adrenoleukodystrophy protein in the human and mouse central nervous system. Neurobiol. Dis. 3 , 271–285 (1997).
Gould, S.J., Krisans, S., Keller, G.A. & Subramani, S. Antibodies directed against the peroxisomal targeting signal of firefly luciferase recognize multiple mammalian peroxisomal proteins. J. Cell Biol. 110, 27–34 ( 1990).
Mihalik, S.J. Et al. Identification of PAHX, a Refsum disease gene. Nature Genet. 17, 185–189 (1997).
Ohba, T. Et al. The structure of the human sterol carrier protein X/sterol carrier protein 2 gene (SCP2). Genomics 24, 370– 374 (1994).
Sakai, Y. Et al. The Candida boidinii peroxisomal membrane protein Pmp30 has a role in peroxisomal proliferation and is functionally homologous to Pmp27 from Saccharomyces cerevisiae. J. Bacteriol. 177, 6773–6781 (1995).
Erdmann, R. & Blobel, G. Giant peroxisomes in oleic acid-induced Saccharomyces cerevisiae lacking the peroxisomal membrane protein Pmp27p. J. Cell Biol. 128, 509– 523 (1995).
Ram, Z. Et al. Growth inhibition, tumor maturation, and extended survival in experimental brain tumors in rats treated with phenylacetate. Cancer Res. 54, 2923–2927 (1994).
Moser, H.W. & Moser, A.B. In Techniques in Diagnostic Biochemical Genetics: A Laboratory Manual (ed. A., H.F.) 177– 191 (Wiley-Liss, New York, 1991).
Watkins, P.A., Ferrell, E.V., Jr., Pedersen, J.I. & Hoefler, G. Peroxisomal fatty acid beta-oxidation in HepG2 cells. Arch. Biochem. Biophys. 289, 329–336 ( 1991).
Zenger-Hain, J., Craft, D.A. & Rizzo, W.B. In New Developments in Fatty Acid Oxidation (eds Coates, P.M. & Tanada, K.) 339–407 (Wiley-Liss, New York, 1992).
Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970).

Comments
Post a Comment